mass of an electron in amu|proton neutron electron mass amu : Cebu Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are relatively heavy particles with no charge and a mass of .
Hayden Kho Sex Sarap Maricar Reyes (PART 2) - SarapBabe on SarapBabe, SarapBabe is home to the widest selection of the best sex videos, pinay porn amateur filipino porn.
mass of an electron in amu,The symbol of an electron is e-. Mass of electron in amu (atomic mass unit): The mass of an electron in amu is determined by the mass of an electron (in Kg) divided by the mass of 1 amu. Mass of electron = 9. 109 × 10-31 Kg; Mass of 1 amu = 1. 66 × 10-27 Kg; . Mass of electron. Values . mass of electron in kg. 9.10938356(11) × 10 −31. kg. mass of electron in grams. 9.10938356(11) × 10 −28. grams. mass of electron in .
Explanation: The mass of an electron is listed as being equal to. melectron = 9.10938356 ⋅ 10−31kg. The unified atomic mass unit, or u, is defined as the mass of one .In particle physics, the electron mass (symbol: me) is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics. It has a value of about 9.109×10 kilograms or about 5.486×10 daltons, which has an energy-equivalent of about 8.187×10 joules or about 0.511 MeV.

Mar 23, 2023
Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are relatively heavy particles with no charge and a mass of .
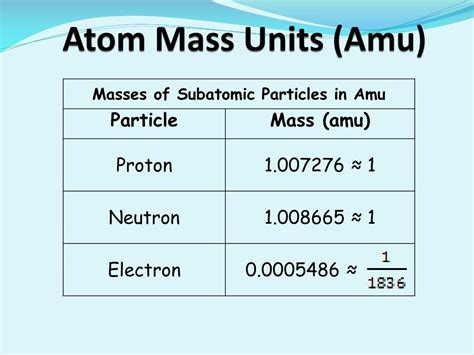
Mass (amu) Relative Mass (proton = 1) Relative Charge Location; proton: p + 1: 1 +1: inside the nucleus: electron: e −: 5.45 × 10 −4: 0.00055: −1: outside the .Mass (amu) Relative Mass (proton = 1) Relative Charge Location; proton: p + 1: 1 +1: inside the nucleus: electron: e −: 5.45 × 10 −4: 0.00055: −1: outside nucleus: neutron: n 0: 1: 1: . Answer link. 9.110*10^-28" g"="0.000549 amu" Source: https://cbc-wb01x.chemistry.ohio-state.edu/~woodward/ch121/ch2_atoms.htm Note: Electrons are .mass of an electron in amuMass: electron : e--1 : 0.0005486 amu : proton : p + +1 : 1.007276 amu : neutron : n o: 0 1.008665 amu
proton neutron electron mass amu⚖️ Atomic Mass Units. An atomic mass unit (amu) is defined as one-twelfth of the mass of a carbon-12 atom, which is about 1.660 × 10 −27 kg. Atomic mass units (amu) are useful, since the mass of a proton and the mass of a neutron are almost equal to 1 . Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can .The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly of the mass of a carbon-12 atom and is equal to 1.6605 10 −24 g. Protons are . Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can .
Therefore, as mentioned, mass of an electron in amu = 9.1 × 10−31 1.66 × 10−27 9.1 × 10 − 31 1.66 × 10 − 27 = 0.00055 u (approximately). Thus, the mass of an electron in amu is 0.00055 u. The correct option is (A). Note: Don’t get confused with the calculation of the value of one atomic mass unit in kilograms.
What is the mass of an electron in amu? Chemistry Matter Basic Atomic Structure. 1 Answer mimi .The invariant mass of an electron is approximately 9.109 × 10 −31 kilograms, or 5.489 × 10 −4 atomic mass units. Due to mass–energy equivalence, this corresponds to a rest energy of 0.511 MeV (8.19 × 10 −14 J). The ratio between the mass of a proton and that of an electron is about 1836. Masses for the three subatomic particles can be expressed in amu (atomic mass units) or grams. For simplicity, we will use the amu unit for the three subatomics. Both neutrons and protons are assigned as having masses of 1 amu each. In contrast, the electron has a negligible mass of .0005 amu.
1 amu = 1.67×10 -24 grams. Mass of electrons = 9 .1 × 10 − 28 1 .67 × 10 − 24 = 5 .4 × 10 − 4 amu.
The electronic configuration of a dipositive metal is 2, 8, 14 and its atomic mass is 56 amu. The number of neutrons in its nuclei would be: A bivalent metal has an equivalent mass of 32. The molecular mass of the metal nitrate is (in amu): An ion having a 4+ charge and a mass of 51.99 amu has two electrons with n=1, eight electrons with n=2 .mass of an electron in amu proton neutron electron mass amu A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu (it would take about 1800 electrons to equal the mass of .The mass of the neutron is greater than that of the proton by 1.293 32 MeV/c 2, hence the neutron's mass provides energy sufficient for the creation of the proton, electron, and anti-neutrino. In the decay process, the proton, electron, and electron anti-neutrino conserve the energy, charge, and lepton number of the neutron.The formal definition of the Atomic Mass Unit (AMU) is 1/12 the mass of a carbon-12 atom. Less formally, the AMU is the average of the rest mass of the protons and neutrons in an atom.Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 × 10 −23 g, and an electron has a charge of less than 2 × 10 −19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) .

A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu (it would take about 1800 electrons to equal the mass of one proton).One unified atomic mass unit(amu) is defined as being equal to 1/ 12 th of the mass of a carbon-12 atom. We know, that the mass of an electron is given as; M electron = 9. 10938356 × 10 − 31 kg; Also, we have, have 1 amu = 1. 660538922 × 10 − 27 kg; Hence, the mass of an electron expressed in unified atomic mass units will be calculated as;
The atomic mass unit (u or amu) is a relative unit based on a carbon-12 atom with six protons and six neutrons, which is assigned an exact value of 12 amu's (u's). This is the standard unit for atomic or molecular mass, and 1 amu is thus 1/12 th the mass of a 12 C atom. This is obviously very small. 1 amu = 1.66054x10-27Kg = 1.66054x10-24 g.
mass of an electron in amu|proton neutron electron mass amu
PH0 · the mass of a proton
PH1 · proton neutron electron mass amu
PH2 · mass of proton in amu
PH3 · mass of electron in u
PH4 · mass of an electron in kg
PH5 · mass of a single electron
PH6 · formula mass in amu
PH7 · electron rest mass
PH8 · Iba pa